Chromatography is a method widely used. You can use it in pharmaceutical and clinical testings, for food and beverage analyses, testing for drugs, in forensics… the list goes on and on.
To fully understand chromatography and to make correct and detailed analyses, you have to have all the concepts and parts up your sleeve. There isn’t a best way to learn all of it than just starting. Step-by-step.
In this article, find out all about retention factor/capacity factor/K-prime in chromatography. Here, you will find information about the sole definition, how to change capacity factors, the effects on resolution, and more. Let’s dig in!
Capacity Factor Or K-Prime: The Definition
In chromatography, the Capacity Factor is a computation or formula that determines how much contact the solute (the other name is a sample peak) has with the stationary phase materials. Or the relative time interacting with the support vs. the mobile phase.
If this contact is too brief, no chromatography has occurred, and you have instead invented a “flow-injection” approach. That is an approach without the need for a column.
The contact should be long enough to prove that the proposed approach is unique to the sample. Also, it should be long enough to have high selectivity for the samples under consideration.
You must follow the fundamentals of chromatography in general in order to design high-quality HPLC procedures. The retention of the sample(s) on the HPLC column utilized and not rinsed out at or near the column void volume is the highest on this list of essentials for most types of HPLC separation. The time of separation in minutes is called “T-zero”.
Although it may seem self-evident at first, many chromatographic procedures fail this retention test and are thus disabled. So naturally, you don’t want that happening to you!
The capacity factor of a sample allows us to be sure that it is retained and eluted past the crucial threshold. But, to calculate it, we must first know the column’s void volume.
The retention period of the unretained sample and the K prime of each sample eluted into it can be calculated, as we said above. Do this using the empty volume of the column. Voila!
If your HPLC technique does not keep the sample on the column for long enough beyond this period, no chromatography can take place.
There is also a K-prime formula that you can use in your research, and we will talk about it more but later in this article.
k1 = [T (R) – T (0)] / T (0)
Keep reading for more information!
How To Change Capacity Factor And What Are The Effects On Resolution?
First, let’s talk about how to change the capacity factor.
If you want to change a peak’s retention factor, you must adjust the chromatographic mobile phase’s solvent strength. This is the most efficient and helpful approach to changing a peak’s retention factor.
If you want to change a peak’s retention actor in reversed-phase chromatography, do that by adjusting the quantity of organic solvent in the mobile phase mixture. This is a common way of changing a peak’s retention factor in reversed-phase chromatography.
A 10% enhancement in the organic material of the mobile phase reduces K by a ratio of two to three for each component.
Changing the mobile phase composition is a powerful separation technique when improving HPLC separations.
Here is a chart that shows the effect on K values of changing mobile phase organic composition:
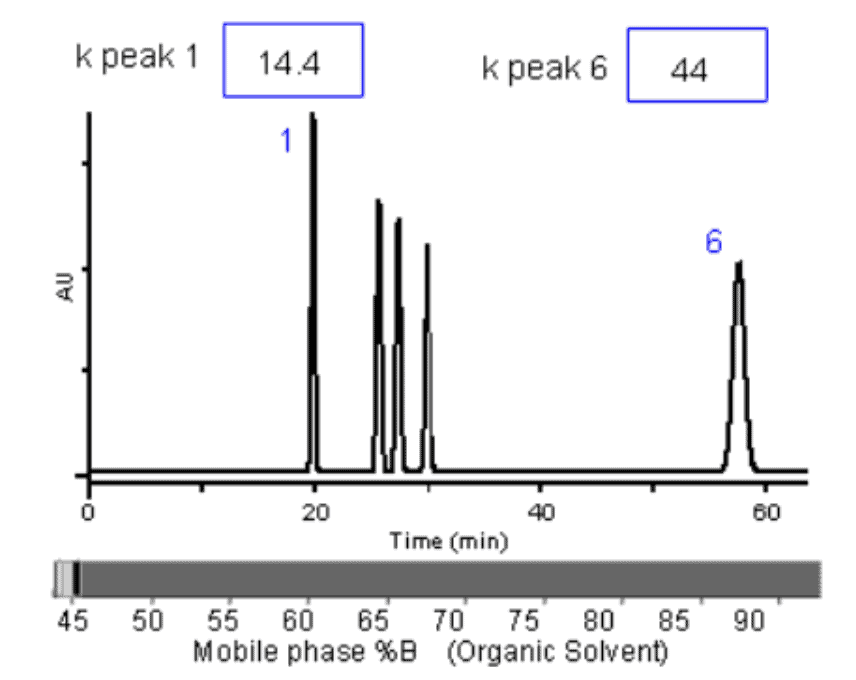
Source: CHROMacademy
When retention factors are extremely high or extremely low, the separation quality suffers. Retention factors of less than one for any analytes usually state poor separation.
The Basics To Know About Capacity Factor
We will generally talk about the retention factor, and if you have any questions, feel free to get back to this chapter as many times as you need!
K-Prime In Chromatography
First, we want to tell you how to find a “K” in the capacity factor. Generally, the capacity factor means how long a compound can be kept in the stationary phase. It’s computed as k = (Tr – To)/To, where “Tr” is the target’s retention time and “To” is the peak time that hasn’t been retained. So the sample is not maintained by the stationary phase if Tr = To.
We already mentioned the formula, but let’s get into it further.
This is the formula (the same one stated above): k1 = [T(R) – T(0)] / T(0). In it, the T(R) equals the retention time of the peak in minutes and T(0) is K of your sample. It has to be more than 1.00, and somewhere around 1.5 is excellent, which should be your goal.
To fully understand what is going on, here is a good video for all who want to know more!
Now let’s talk about what is a good retention/capacity factor!
What Is A Good Retention/Capacity Factor/K-Prime?
As stated before, the retention factor (K) is a metric for determining how long an analyte will stay on a chromatographic column. The retention factor is the analyte’s column retention time to the non-retained compound’s retention time.
In chromatography, the capacity factor defines the contact with the sorbent or retention. A K of 1 or greater is required for the earliest eluting peak of interest. A score of two or above is excellent.
The most straightforward technique to raise a solute’s retention factor in liquid chromatography is to employ a weaker mobile phase. Solutes spend correspondingly longer in the stationary phase and take longer to elute when the mobile phase has a lower solvent strength.
Follow this if you want to raise the retention factor:
- Use solar panels with a high-efficiency rating of > 18%.
- Use batteries to provide power 24 hours a day, seven days a week which can boost the CF by 50 percent to 100 percent.
- Use tracking devices.
Logically, if the retention actor is not high enough, it is low. You may ask yourself why?
Well… There are several reasons why a plant’s capacity factor is less than 100%. These include technical restrictions such as plant availability, economic considerations, and energy resource availability.
Here is another video about the retention factor, and it shows fundamentals and all the things you should know before engaging in research!
The Role Of Capacity Factor In The Selectivity In Chromatography
In the short version, the selectivity (or separation) factor is the capacity of the chromatographic system to differentiate between sample components chemically.
It’s often calculated as a ratio of the capacity factors of the two summits in question, and it’s displayed as the distance between their apices.
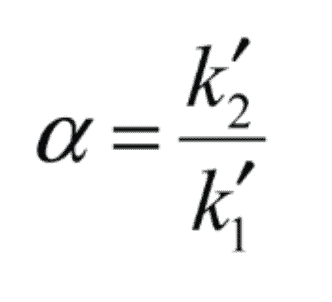
Source: Restek
High numbers of the Alpha imply high separating power and separation between each peak’s APEX. The resolution is not exactly proportional to the alpha value.
Because when it is equal to one, the selectivity is always larger than one (i.e., their capacity factor values are identical). The apices of the two peaks get farther apart as the selectivity value rises.
The chemistry of the analyte, mobile phase, and stationary phase influences the selectivity of separation. Now, you can change all these variables or optimize them to improve the selectivity of an HPLC separation.
While other separation modes may be employed to influence how analytes interact with a specific stationary phase, we will now focus on RP separations.
RP separations are something that almost every science student has done before. However, the first thing to remember is that not all C18* phases are the same.
The retention of various analytes is affected by changes in the underlying support chemistry, the way bonded groups are linked to the support, and how potentially detrimental interactions with leftover silanol groups are protected.
There is so much more to say about the selectivity factor, but let’s stop here and let you infuse all the information.
*A C18 column is somewhat like a “reverse phase”. Water, acetonitrile, and methanol are some of the polar solvents frequently utilized with reverse-phase columns.
Conclusion
Understanding the capacity factor in the chromatography world can take you high on the productivity scale.
Knowing that is most important if you want to do your research correctly and in detail. Therefore, it is not something that can be overlooked.
Hopefully, this article brought the answers to your questions. Check other blogs as well to understand chromatography and its usage fully.
Frequently Asked Questions
If you didn’t get all the needed information above, here is a quick FAQ. Hopefully, here is the answer you’re looking for!
What Does It Imply To Have A Low Retention Factor?
We’ve previously answered this question, but it appears to be the most often requested, so let’s wrap it up with this definition.
A high retention factor indicates a significant interaction between the molecule of interest and the surface. Conversely, the interaction between the chemical of interest and the surface is poor if the retention factor is low.
In Chromatography, What Is The Rf Factor?
In chromatographic separation, the retardation factor (Rf) is the ratio of the distance traveled by the material of interest to the distance traveled simultaneously by the mobile phase. It is usually always less than 1.
The Rf values demonstrate how soluble the pigment is in the solvent by measuring how high the pigment travels on the paper. It’s almost clear that two pigments with the same Rf value are the same molecule. Small Rf values imply pigments that are more significant but less soluble, whereas Rf values around one indicate pigments that are very soluble.