Introduction
Have you ever wondered how chromatography techniques separate different components in a sample? One of the key factors that determine this separation is the retention factor (Rf). Whether you’re just starting to explore chromatography or have years of lab experience, understanding how retention factors work is essential for accurate analysis. What do you think of that? Does the term “retention factor” seem a bit abstract? Let’s break it down together!
In this article, we’ll explore the formula of the retention factor, why it’s important, and how it impacts the results you get in chromatography.
What is Retention Factor (Rf)?
Chromatography is a technique used to separate mixtures based on the different affinities of compounds to a stationary and a mobile phase. The retention factor, or Rf value, is a numerical value that indicates how far a compound travels relative to the solvent front.
So, what does that mean in practical terms?
Formula of Retention Factor (Rf)
The retention factor is calculated using the following formula:

Now, let’s break this down a bit:
- Distance traveled by the compound: This is the distance from the baseline (where the sample was initially spotted) to the center of the spot of the compound you’re analyzing.
- Distance traveled by the solvent front: This is the distance from the baseline to the furthest point that the solvent has traveled.
The Rf value is always between 0 and 1. An Rf of 0 means the compound didn’t move at all (it’s stuck at the origin), and an Rf of 1 means the compound traveled exactly as far as the solvent front.
How Do You Use the Retention Factor in Chromatography?
Now, you might be wondering: How is the Rf value useful?
Here’s the thing: Rf values are used to identify compounds. While each compound may have a unique Rf value under specific conditions (like temperature and solvent), this value can act as a fingerprint, helping you identify substances when compared to known standards.
Factors That Affect Rf Value
Your experiment’s environment can have a big impact on the Rf value of a compound. Let’s look at some key factors:
- Type of solvent: The polarity of the solvent will influence how far different compounds move. In fact, solvents can be adjusted to improve the separation of components.
- Stationary phase: The material that makes up the stationary phase (like silica gel) plays a crucial role in how compounds interact and move.
- Temperature: The rate of movement of compounds can change with temperature. A higher temperature can cause molecules to move faster, altering their Rf values.
- Concentration of the sample: A high concentration of a sample might create broader spots, making it harder to determine an accurate Rf value.
Why is the Retention Factor Important?
Understanding Rf is more than just a fun lab exercise—it’s a key factor in ensuring the accuracy of chromatographyresults. If you have a low Rf value, it could indicate poor separation, or that the compound isn’t interacting strongly with the stationary phase. High Rf values, on the other hand, suggest that compounds are not being retained effectively and could be eluting too quickly.
Think about this: What happens if your Rf values are inconsistent across different trials? That could mean your method isn’t as reliable as it should be, and you might need to tweak something in your setup. Rf helps in troubleshooting chromatography methods, identifying compounds, and confirming the efficiency of your separation process.

Types of Chromatography and Their Use of Rf
Now, it’s interesting to note that Rf values are used in several types of chromatography, including thin-layer chromatography (TLC), paper chromatography, and liquid chromatography (HPLC).
Let’s dive into each:
Thin-Layer Chromatography (TLC)
In TLC, the stationary phase is a thin layer of adsorbent material spread on a plate. The sample is spotted onto this layer, and then a solvent is used to develop the chromatogram. The Rf value helps determine the purity and identity of the sample.
Paper Chromatography
In paper chromatography, the stationary phase is simply filter paper. Similar to TLC, the Rf value tells you about the solubility and polarity of the compound in the mobile phase. Do you see how different chromatography techniques use Rf values in slightly different ways? It’s pretty neat how versatile this number is!
Liquid Chromatography (HPLC)
While HPLC uses columns and pumps, the principle is still rooted in separating compounds based on their different interactions with the stationary phase. Retention times in HPLC can be used similarly to Rf values in TLC, but are often interpreted alongside other data like peak areas and widths.
Practical Example: Calculating the Retention Factor
Let’s say you’re conducting a TLC experiment, and after developing the chromatogram, you measure:
- The distance traveled by the solvent front: 10 cm
- The distance traveled by your compound: 4 cm
Using the formula:
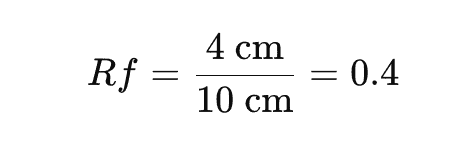
This means your compound has an Rf of 0.4 in the given solvent system.
What do you think of this approach? Would you use this method in your own experiments?
Common Mistakes to Avoid
Now that we’ve covered the basics of the retention factor, let’s take a look at some common mistakes people make when working with Rf values:
- Not measuring accurately: Small errors in measuring distances can lead to significant inaccuracies in your Rf calculation. Make sure you measure carefully.
- Ignoring environmental factors: As we discussed earlier, things like solvent choice, temperature, and sample concentration can all affect the Rf value. Always control your experimental conditions as much as possible.
- Assuming Rf values are always the same: Keep in mind that Rf values can change depending on the chromatographic system. If you change the solvent or column, the Rf value may not be the same.
Conclusion
In summary, understanding the formula for retention factor and how to calculate it is crucial for anyone working with chromatography. Whether you’re separating compounds in the lab or analyzing complex mixtures, your retention factor can help guide your analysis and ensure accuracy. It’s a simple but powerful tool in the world of chromatography.
What do you think about the role of retention factor now? Does it make more sense? Let me know your thoughts or any questions you might have!
Here are some of the most relevant sources I consulted:
- High Performance Liquid Chromatography – Chemistry LibreTexts
This article explains the fundamentals of HPLC, including details about retention factors, selectivity, and separation efficiency. It also covers essential concepts like the distribution constant, retention time, and peak resolution that are crucial when discussing how HPLC vials impact chromatographic separations. - Optimizing Chromatography Systems for Accurate Results
Read about the best practices for improving your chromatography system and ensuring accurate results, focusing on factors that affect Rf values.