Introduction: The Impact of pH on Retention, Selectivity, and Peak Shape
When developing an HPLC method, one of the most critical factors influencing separation is pH. Whether you’re analyzing pharmaceuticals, biomolecules, or food components, pH affects analyte ionization, retention time, selectivity, and peak shape. Poor pH control can lead to broad peaks, retention time shifts, and poor reproducibility, making your method unreliable.
The right buffer system helps maintain a stable pH environment, ensuring consistent separations. However, choosing an inappropriate buffer—or failing to control pH—can lead to poor resolution, column damage, and irreproducible data.
In this guide, we’ll explore:
✔ How pH influences analyte ionization and retention
✔ How to select the best buffer system for your method
✔ Preventing column degradation from pH extremes
✔ Best practices for pH control and method robustness
✔ A case study on pH optimization in pharmaceutical analysis
By the end, you’ll understand how to optimize pH and buffer selection to achieve accurate, reproducible HPLC results.
1. Understanding the Influence of pH on Analyte Ionization
1.1 Why Does pH Matter in HPLC?
pH directly affects:
✔ Retention Time – Ionized compounds interact less with the stationary phase, eluting faster.
✔ Selectivity – Adjusting pH changes relative retention of different analytes.
✔ Peak Shape – Poor pH control can cause peak tailing or splitting.
1.2 The Henderson-Hasselbalch Equation
To predict how pH affects ionization, use the Henderson-Hasselbalch equation:
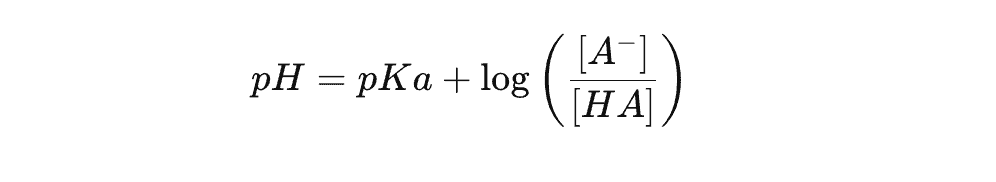
Where:
✔ pKa = The dissociation constant of the analyte.
✔ HA = The protonated (neutral) form.
✔ A⁻ = The ionized form.
1.3 pH and Retention of Acidic and Basic Compounds
✔ Acidic compounds (e.g., ibuprofen, benzoic acid)
- At pH < pKa, acids are neutral → Higher retention.
- At pH > pKa, acids are ionized → Lower retention.
✔ Basic compounds (e.g., amines, caffeine)
- At pH > pKa, bases are neutral → Higher retention.
- At pH < pKa, bases are ionized → Lower retention.
Choosing the right pH range (typically pKa ± 1) allows for optimal retention and selectivity.
2. Choosing the Right Buffer System for Your Method
Buffers help maintain a stable pH, preventing retention shifts during analysis.
2.1 Selecting the Best Buffer Based on pH Range
| Buffer System | pKa Range | Suitable for |
|---|---|---|
| Phosphate (NaH₂PO₄ / Na₂HPO₄) | 2.1, 7.2 | Reversed-phase HPLC |
| Acetate (Acetic Acid / Sodium Acetate) | 4.8 | Organic acids, pharmaceuticals |
| Formate (Formic Acid / Ammonium Formate) | 3.8 | LC-MS applications |
| Citrate (Citric Acid / Sodium Citrate) | 3.1, 4.7, 5.4 | Biological samples |
| Trifluoroacetic Acid (TFA) | 0.3 | Peptide separations |
2.2 Buffer Considerations for Different Detection Methods
✔ UV Detection: Avoid buffers with high UV absorbance (e.g., phosphate below 210 nm).
✔ Mass Spectrometry (LC-MS): Use volatile buffers (e.g., formate, acetate).
✔ Ion-Exchange Chromatography: Choose buffers that stabilize charge interactions.
2.3 Buffer Strength and Ionic Strength Effects
✔ Higher buffer concentration (10–50 mM) improves pH stability but may increase backpressure.
✔ Lower ionic strength can lead to retention time shifts in gradient elution.
Choosing the correct buffer type, concentration, and pH ensures stable, reproducible separations.
3. How to Prevent Column Degradation Due to pH Extremes
3.1 pH Limits for HPLC Columns
✔ Silica-Based Columns (C18, C8, Phenyl) → pH 2–8 (above pH 8, silica dissolves!).
✔ Hybrid or Polymer Columns (BEH, PEEK, Zirconia) → pH 1–12, better for extremes.
3.2 Risks of Using pH Extremes
🚩 Low pH (<2):
- Damages silica columns, leading to loss of retention.
- TFA at low pH may cause excessive peak tailing.
🚩 High pH (>8):
- Hydrolyzes silica bonds, reducing column lifetime.
- Causes irreversible analyte adsorption.
3.3 How to Extend Column Life
✔ Use Hybrid or Polymer-Based Columns for stability at pH extremes.
✔ Flush columns regularly with neutral solvents (e.g., 50:50 methanol/water).
✔ Avoid prolonged storage of columns in extreme-pH mobile phases.
Proper pH management prevents early column failure and inconsistent results.

4. pH and Method Robustness: Best Practices
4.1 Controlling pH for Reproducibility
✔ Prepare fresh buffers daily to avoid degradation.
✔ Use pH meters calibrated with high-precision standards.
✔ Verify pH before filtration—filtration can slightly alter buffer pH.
4.2 pH and Batch-to-Batch Consistency
✔ Maintain the same buffer concentration across batches.
✔ Check mobile phase pH stability after mixing organic solvents.
4.3 Using pH Gradients in Method Development
✔ Stepwise pH gradients can improve peak resolution in complex separations.
✔ pH-programmed HPLC helps optimize selectivity for ionizable compounds.
Implementing pH control best practices ensures a robust, reliable HPLC method.
5. Case Study: pH Optimization in Pharmaceutical Analysis
5.1 Problem
A pharmaceutical lab struggled with poor resolution and retention time variability in the analysis of a weakly basic drug and its impurities.
5.2 Investigation
🔎 Original Conditions:
- Column: C18 (pH range 2–8)
- Mobile Phase: Acetonitrile/water (pH 7.2, phosphate buffer)
- Issues: Peak tailing, retention shifts between batches.
5.3 Solution
✔ Adjusted buffer pH to 3.5 (closer to the drug’s pKa).
✔ Switched to a volatile formate buffer for better peak shape.
✔ Used a hybrid silica column (pH 1–12 stable) to prevent degradation.
5.4 Results
✔ Baseline-separated peaks with Rs > 2.0.
✔ Retention time variation reduced from ±5% to <1%.
✔ Increased column lifespan by 30%.
This case study shows how pH optimization enhances resolution, reproducibility, and column stability.
Conclusion: Key Takeaways on Buffer and pH Selection
To achieve better HPLC separations, follow these pH optimization principles:
✔ Choose the right buffer – Match buffer pKa with analyte pKa.
✔ Control pH within column limits – Stay within pH 2–8 for silica columns.
✔ Optimize buffer concentration – Use 10–50 mM for stability.
✔ Prevent column damage – Use hybrid columns for extreme pH.
✔ Calibrate pH meters and prepare fresh buffers to ensure reproducibility.
Mastering pH control will significantly improve your HPLC method robustness and accuracy. Are you ready to fine-tune your mobile phase for better resolution and peak shape? 🚀
Mastelf, with over 13 years of experience in chromatography vials, we can help you find the exact vials you need for your applications.


Our expertise ensures that you get reliable and precise products tailored to your specific requirements. Whether you’re in pharmaceuticals, research, or any other industry relying on HPLC, we understand your needs and are here to support you in making the right purchase.
Reach out to Mastelf, and let us assist you in procuring the perfect vials for your work.
FAQs
1. How does pH affect peak shape?
pH influences analyte ionization, which affects retention, peak width, and symmetry.
2. What happens if I use pH 9 on a silica-based column?
Silica dissolves at high pH, leading to column degradation and retention loss.
3. Why does my buffer pH change after mixing with organic solvents?
Organic solvents alter buffer dissociation, causing pH shifts—always check after mixing.











