Introduction: Why Resolution is Critical for Accurate Results
If you’ve ever struggled with overlapping peaks in your HPLC chromatograms, you’re not alone. Resolution (Rs) is one of the most critical parameters in High-Performance Liquid Chromatography (HPLC) because it determines how well two analytes are separated. Poor resolution can lead to incorrect quantification, loss of critical information, and regulatory compliance issues—a nightmare for any analyst.
But what exactly affects HPLC resolution, and how can you improve it? Factors like mobile phase composition, column selection, flow rate, and temperature all play a role in enhancing separation. Even minor adjustments to these parameters can turn an unusable chromatogram into a high-resolution, publication-quality result.
In this guide, we’ll cover:
✔ What defines resolution in HPLC?
✔ How to optimize mobile phase and gradients
✔ Choosing the best column and particle size
✔ How flow rate and temperature impact resolution
✔ Real-world techniques for improving separation
By the end, you’ll have practical strategies to maximize resolution and achieve clear, well-separated peaks in your analyses.
1. What Defines Resolution in HPLC?
Understanding HPLC Resolution (Rs)
HPLC resolution (Rs) is the ability to distinguish between two adjacent peaks in a chromatogram. It is mathematically defined as:
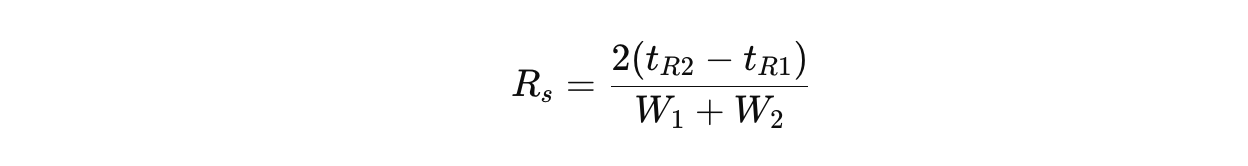
Where:
✔ tR1 and tR2 = Retention times of two peaks
✔ W1 and W2 = Peak widths at baseline
A resolution of Rs ≥ 1.5 is considered sufficient for baseline separation.
Three Key Factors Affecting Resolution
According to the Resolution Equation:
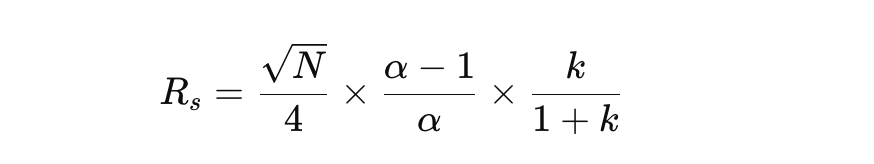
1️⃣ Efficiency (N) – Controlled by column length and particle size.
2️⃣ Selectivity (α) – Controlled by mobile phase composition and column chemistry.
3️⃣ Retention Factor (k’) – Controlled by solvent strength and gradient settings.
Now that we understand what defines resolution, let’s dive into how to optimize key parameters for better separation.
2. Optimizing Mobile Phase Composition and Gradients
2.1 Adjusting Solvent Strength for Better Resolution
✔ Increase Organic Modifier (e.g., acetonitrile, methanol) → Reduces retention times, but may lower resolution.
✔ Increase Aqueous Phase → Increases retention, allowing better peak separation.
✔ Use Buffering Agents (e.g., phosphate, formic acid) → Controls ionization of analytes, improving peak shape.

2.2 Fine-Tuning pH for Optimal Selectivity
✔ For acidic compounds (pKa < 5) → Use low pH (< 3.5) to suppress ionization.
✔ For basic compounds (pKa > 7) → Use higher pH (7–10) to improve retention.
✔ Avoid pH extremes that can degrade the column.
2.3 Optimizing Gradient Elution for Complex Samples
✔ Shallower Gradient (e.g., 10% to 60% organic in 20 min) → Better resolution for closely eluting peaks.
✔ Steeper Gradient (e.g., 10% to 90% in 5 min) → Faster run times, but lower resolution.
✔ Isocratic vs. Gradient – Gradient elution improves separation for analytes with different polarities.
By tweaking the solvent composition and gradient profile, you can achieve higher resolution and sharper peaks.
3. Choosing the Right Column and Particle Size
3.1 Column Length and Internal Diameter
✔ Longer Columns (150–250 mm) → Higher plate numbers (N), improving resolution.
✔ Narrower Columns (2.1 mm ID) → Higher sensitivity but may require lower flow rates.
3.2 Selecting the Best Particle Size
✔ Smaller Particles (e.g., 1.7 µm in UHPLC) → Increase efficiency (N) and resolution.
✔ Larger Particles (5 µm) → Lower backpressure but may reduce separation power.
✔ Sub-2 µm (UPLC) – Best for fast, high-resolution separations.
3.3 Choosing the Right Stationary Phase
✔ C18 Columns → Ideal for hydrophobic, non-polar compounds.
✔ C8 Columns → Shorter retention times, good for moderate polarity compounds.
✔ Phenyl, HILIC, and Ion-Exchange Columns → Best for specialized separations (e.g., polar, charged molecules).
Column selection is critical—a simple switch from C18 to a phenyl column can drastically improve selectivity.

4. Effect of Flow Rate and Temperature on Resolution
4.1 Flow Rate Adjustments
✔ Lower Flow Rate (e.g., 0.5 mL/min) → Increases resolution, but longer run times.
✔ Higher Flow Rate (e.g., 1.5 mL/min) → Faster runs, but lower peak separation.
✔ Optimal Flow Rates:
- 1.0 mL/min for standard HPLC (4.6 mm ID)
- 0.3–0.5 mL/min for UHPLC (2.1 mm ID)
4.2 Temperature Effects on Peak Shape
✔ Increasing Temperature (e.g., 30°C to 50°C) →
- Decreases mobile phase viscosity → better mass transfer.
- Improves separation for highly retained compounds.
✔ Too High Temperatures (>60°C) → Can degrade thermolabile compounds.
Fine-tuning flow rate and column temperature can significantly enhance resolution.
5. Real-World Examples of Improved Resolution Techniques
Case Study 1: Optimizing a Drug Impurity Profile
🚩 Problem: Impurity peaks overlapped with the main drug peak.
🔹 Solution: Switched from C18 to a phenyl column + adjusted pH.
✔ Outcome: Baseline resolution (Rs > 1.5) achieved.
Case Study 2: Resolving Sugars in Food Analysis
🚩 Problem: Glucose and fructose peaks merged.
🔹 Solution: Used HILIC column + increased organic solvent (80% acetonitrile).
✔ Outcome: Full separation with sharp, well-defined peaks.
Case Study 3: Improving Resolution in Peptide Analysis
🚩 Problem: Poor resolution of peptide fragments.
🔹 Solution: Switched from isocratic to gradient elution (10–50% ACN).
✔ Outcome: Peptides eluted separately with improved peak sharpness.
These examples highlight how small adjustments can lead to major improvements in resolution.
Conclusion: Summary of Techniques for Better Separation
Improving HPLC resolution requires fine-tuning multiple parameters:
✔ Optimize Mobile Phase – Adjust organic solvent, pH, and gradients.
✔ Choose the Right Column – Select optimal length, particle size, and stationary phase.
✔ Control Flow Rate & Temperature – Adjust to enhance efficiency without sacrificing speed.
✔ Apply Advanced Techniques – Consider UPLC, HILIC, and alternative phase chemistries.
By implementing these strategies, you can ensure clear, high-resolution peaks, improving both data quality and analytical confidence. 🚀
FAQs
1. What is the best way to improve resolution in HPLC?
Adjust mobile phase composition, use a smaller particle column, and optimize flow rate.
2. How does pH affect resolution?
pH impacts ionization of analytes, influencing retention and peak shape.
3. Should I use isocratic or gradient elution for better resolution?
Gradient elution is better for complex mixtures, while isocratic works well for single-compound analyses.
4. How does temperature impact resolution?
Higher temperatures improve mass transfer, but can degrade heat-sensitive analytes.
5. What is the best column for maximum resolution?
A longer column with smaller particles (e.g., UPLC with 1.7 µm particles) provides the highest efficiency.