Distillation is a topic I have encountered in my younger years in school. Gas chromatography is something I learned at the secondary level.
The two methods are fundamentally different, but they have something in common.
Essentially, gas chromatography and distillation utilize heat in the process. Although distillation can separate mixtures, it can also deal with compounds. However, gas chromatography is being used to analyze compounds. At the same time, distillation aims to separate components of a compound for practical use.
I am amazed at the distillation and gas chromatography applications, and I’ll share more details with you in this blog. But first, let’s talk about solutions and compounds.
A mix-up between solutions, mixtures, and compounds
The three could be the most basic of topics in Science. Even though they are certainly technically different, it can get quite confusing at times.
Take a look at this very informational video I found from 2 Minute Classroom:
Is a solution a mixture?
When I think about solutions and mixtures, I put it this way: if I can only see one thing on the container, it’s probably a solution. If there are at least two components, it is a mixture.
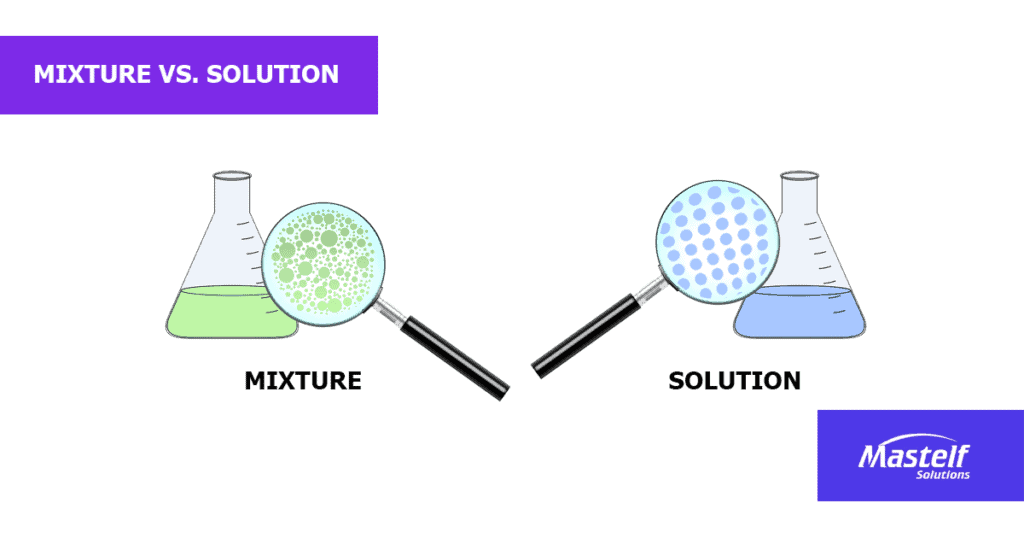
Technically, a solution is also a mixture, only that it is homogeneous such as a salt-water solution, carbonated water, or 70% ethyl alcohol.
There are three primary identities of every solution:
- the liquid allows visible light to pass through
- any of the components cannot be removed from the rest by mechanical filtration
- an individual part can be removed by utilizing its physical properties.
Solutions can be separated using distillation. One of the components has a different boiling point than the other or the rest.
If there is a solid solute such as salt, plain boiling would be acceptable to get the salt. It would be impractical to get the water in a salt solution, only for the salt to stick inside the distillation flask.
I am amazed at how simple fruit extracts can produce alcohol for use as biofuel. In simple experiments, bioethanol can be made from fermented fruit juices.
Distillation can then be performed to separate the liquid-liquid solution. Since ethanol has a boiling point of about 78 degrees Celsius, it will evaporate faster than water.
Practically, you can get the bioethanol out of the solution, but not without a small percentage of water. The idea is that liquids evaporate all the time but in tiny quantities.
You may have also heard about fractional distillation associated with crude oil. Crude oil is a mixture of paraffin, naphthenes, and aromatics, among other components.
Is compound a mixture?
Technically, a compound is not a mixture because the components of a compound are chemically bonded. This means that they cannot be separated by physical means, including distillation.
One or more elements comprise a compound, and it can be one of the following:
- Molecules: more than one atom of the same or different elements are chemically bonded and electrically neutral. An example is a water molecule or an oxygen gas molecule.
- Ionic compound: composed of positively and negatively charged atoms. Examples are sodium cations and chloride anions for table salt.
- Intermetallic compounds: metallic alloy with the solid-state organization.
- Complexes: has a coordination center plus surrounding ions or molecules.
Now, this is where the fun begins. It is possible to get a compound from a given mixture, such as my earlier example of bioethanol from fermented fruit extracts. Of course, water and other solids are also in there that can be separated by mechanical filtration and boiling. But in order to analyze the compound, gas chromatography is needed.
Just because a compound is a distinct sample doesn’t mean that it is pure. For bioethanol, gas chromatography can be used to check for impurities.
How do I know if a substance is a mixture?
In order to determine whether a sample you have is a mixture, you should do some physical experiments.
For example, if you have a clear liquid, you can test its boiling point to know whether it’s water or something else. You may also want to filter it if there are orbeez mixed in, making it a heterogeneous mixture.
The presence of heat can reveal the identity of a sample. If heat applied gives rise to another substance or chemical, the heated sample is a compound. Heat can increase the solubility of a solute in a solution, but taking away heat can make crystals appear in a saturated solution.
What is gas chromatography?
In gas chromatography, of course, we deal with gas. A carrier or mobile phase is used to carry a gas or vaporized liquid sample into a stationary phase. It should be inert such as helium, or unreactive such as nitrogen.
What makes the analysis possible is the relative boiling points of whatever is in the sample. If a compound has a lower boiling point, it will travel faster and farther in the stationary phase. Thus, GC depends entirely on retention times to make a proper analysis.
You must note that the vaporized sample must not decompose or turn into something else because of the heat.
Additionally, GC can be utilized to obtain pure compounds from a mixture as the sample.
Now, suppose we’re working on a compound. In that case, it can be identified based on how it affects the walls of a column containing several stationary phases, all liquid. Hence, the more scientific term for GC is GLPC or gas-liquid partition chromatography. Still, it can also be referred to as vapor-phase chromatography.
We have covered gas chromatography in a previous short article if you want to read more.
Types of gas chromatography
There are two types of gas chromatography, and I’ve simplified them for you:
- GSC or gas-solid chromatography relies on a solid stationary phase where adsorption of the analytes will take place. Therefore, GSC is an adsorption chromatography technique and uses packed columns. It has a longer retention time.
- GLC or gas-liquid chromatography depends on a solvent wherein dissolved molecules or ions are present. So, GLC is a partition chromatography technique and uses capillary columns. It has a shorter retention time which means faster results.
Processes in gas chromatography similar to distillation
For the sake of this scientific discussion, I will consider fractional distillation since it’s the more complex type of distillation.
First, they both have vapors along the process that are crucial to the result. GC needs it for the analysis while it is necessary to obtain a product in distillation.
Second, they both have columns: GC may use capillary columns, while distillation may use short reflux columns.
What is distillation?
Distillation sounds like a pure word to my ear, and it is indeed. You may have heard it for the most common commodity – bottled water. It’s boiling, but the vapor is being collected rather than discarded.
Types of distillation
There are four main types of distillation, although the classifications are not limited to them:
- Simple Distillation is applicable when two liquids have distant boiling points or if the goal is to separate liquid from solids or other components that would not evaporate. A simple setup involves a condenser with running cold water. You can make your own distilled water through this.
- Steam Distillation is applicable when delicate components must not be degraded by heat. Fractions can be produced, such as in the distillation of flowers to obtain essential oils.
- Fractional Distillation is applicable when a mixture has various components with boiling points closer to one another. It utilizes a fractionating column to obtain higher purity samples. Crude oil makes all kinds of industrial and household items through fractional distillation.
- Vacuum Distillation is applicable when respective components of a mixture have boiling points that are way too high. With lower pressure, the boiling point can also be lowered. It is being used in the chemical, food, and pharmaceutical industries to avoid the decomposition of a compound due to higher temperatures.
Processes in distillation similar to gas chromatography
Vapor is an essential part of the process for both distillation and gas chromatography.
Fractional distillation shares more similarities with gas chromatography because it uses columns to separate components.
Vacuum may likewise be used for gas chromatography and vacuum distillation.
Why are gas chromatography and distillation unique from each other?
Gas chromatography and distillation are different because they have respective processes, equipment, and applications.
Don’t be silly to think that just because gas chromatography can be used to obtain purer samples, you can use it to get ultra-purified water. It is for testing and not mass-producing.
Heat plays a significant role in distillation, while it is only incidental to the whole process of gas chromatography.
Moreover, distillation heavily relies on the boiling point of respective compounds. In contrast, gas chromatography, or chromatography in general, depends on the compounds’ polarity that dictates their affinity to both mobile and stationary phases.
Which is superior, gas chromatography or distillation?
I think that there is no single right lens to look through to determine whether gas chromatography or distillation is better than the other.
Each has its applications: gas chromatography helps ensure the purity of samples being analyzed, but distillation can be quite complex to produce results intended for commercial purposes.
They can both work for the benefit of each other, with either being important in their ways. We can compare this to different professions in the world.
Conclusion
Gas chromatography and distillation are different but complementary things useful in analyzing compounds. Mainly, both have impacts way beyond their direct applications. Gas chromatography primarily deals with small samples, but distillation concerns mass production. They can both be used to obtain samples with more purity.
Mastelf can help you with supplies for your gas chromatography experiments. Get in touch today and receive free vial samples.