When you’re dealing with packaging in the pharmaceutical, biotech, or food industry, terms like “dust-free” and “sterilization” often pop up. But what exactly do these mean? I remember my initial confusion about it all—dust-free sounded clean, but sterile sounded like the gold standard. So, let’s dig into it together, just like we would if we were catching up over coffee.
What is Dust-Free Packaging?
Dust-free packaging sounds straightforward, doesn’t it? But there’s more to it. Essentially, it means packaging that prevents dust, dirt, and other particles from contaminating the product. It’s designed to maintain a clean environment, but it doesn’t necessarily keep out bacteria or other microorganisms.
How is Dust-Free Packaging Achieved?
Manufacturers achieve dust-free packaging in a few key ways. First, they use cleanrooms or controlled environments to minimize dust particles in the air. Additionally, the packaging materials themselves are selected for their low-shedding properties. What do I mean by low-shedding? Well, it simply refers to materials that don’t break off or release small particles over time.
For instance, imagine those little silica gel packets in your electronics packaging. These are great for moisture, but if the packaging materials were prone to shedding, it would defeat the purpose. It’s like protecting your electronics from moisture while letting in a bunch of dust—not ideal.
Applications of Dust-Free Packaging
Dust-free packaging is often used in electronics, automotive parts, and non-sterile pharmaceuticals. It’s crucial when dust particles could interfere with a product’s functionality or appearance.
For example, think about delicate computer hardware. Dust can cause overheating or even short circuits, making dust-free packaging essential. It’s not about sterility, but it’s definitely about maintaining a pristine condition.
What is Sterilization?
Now, let’s move on to sterilization. This process is about killing or eliminating all forms of microbial life, including bacteria, viruses, fungi, and spores. Sterilization isn’t just about being clean—it’s about being biologically safe. In medical and pharmaceutical contexts, this is non-negotiable.
Types of Sterilization
Sterilization can be achieved in several ways, including:
- Heat Sterilization: Think of autoclaves—machines that use high pressure and steam to kill microorganisms. It’s a staple in hospitals and labs.
- Chemical Sterilization: This involves using disinfectants or gases like ethylene oxide to eliminate microbial life.
- Radiation Sterilization: This method uses ionizing radiation, which sounds intense (and it is), but it’s a common practice for medical devices.
What’s fascinating is how these methods can be tailored depending on the product. A disposable syringe, for example, wouldn’t be suitable for heat sterilization due to its plastic components. Chemical or radiation sterilization would be the way to go.


Key Differences Between Dust-Free Packaging and Sterilization
Okay, let’s zoom out and compare these two. Dust-free packaging and sterilization share a common goal: protecting the product. But how they do it—and what they protect against—are very different.
The Focus: Particles vs. Microorganisms
Dust-free packaging focuses on eliminating dust, dirt, and particles. It’s all about maintaining a clean physical environment. But, and this is crucial, it doesn’t deal with biological threats like bacteria or viruses.
Sterilization, on the other hand, is all about eliminating these biological contaminants. It’s not just about being clean on the surface—it’s about being safe at a microbial level. I like to think of it as the difference between wiping down a table and disinfecting it.
Reusability and Stability
Another difference is the impact on the product’s reusability. Dust-free packaging materials can often be reused if they’re well-maintained. Sterilization, however, might involve single-use packaging or require specific storage conditions to maintain sterility.
Applications in Different Industries
Dust-free packaging and sterilization are used differently across industries. Dust-free packaging is prevalent in electronics, automotive, and some pharmaceuticals. Sterilization is essential in healthcare, food, and any application where microbial safety is a top concern.
Choosing the Right Option for Your Needs
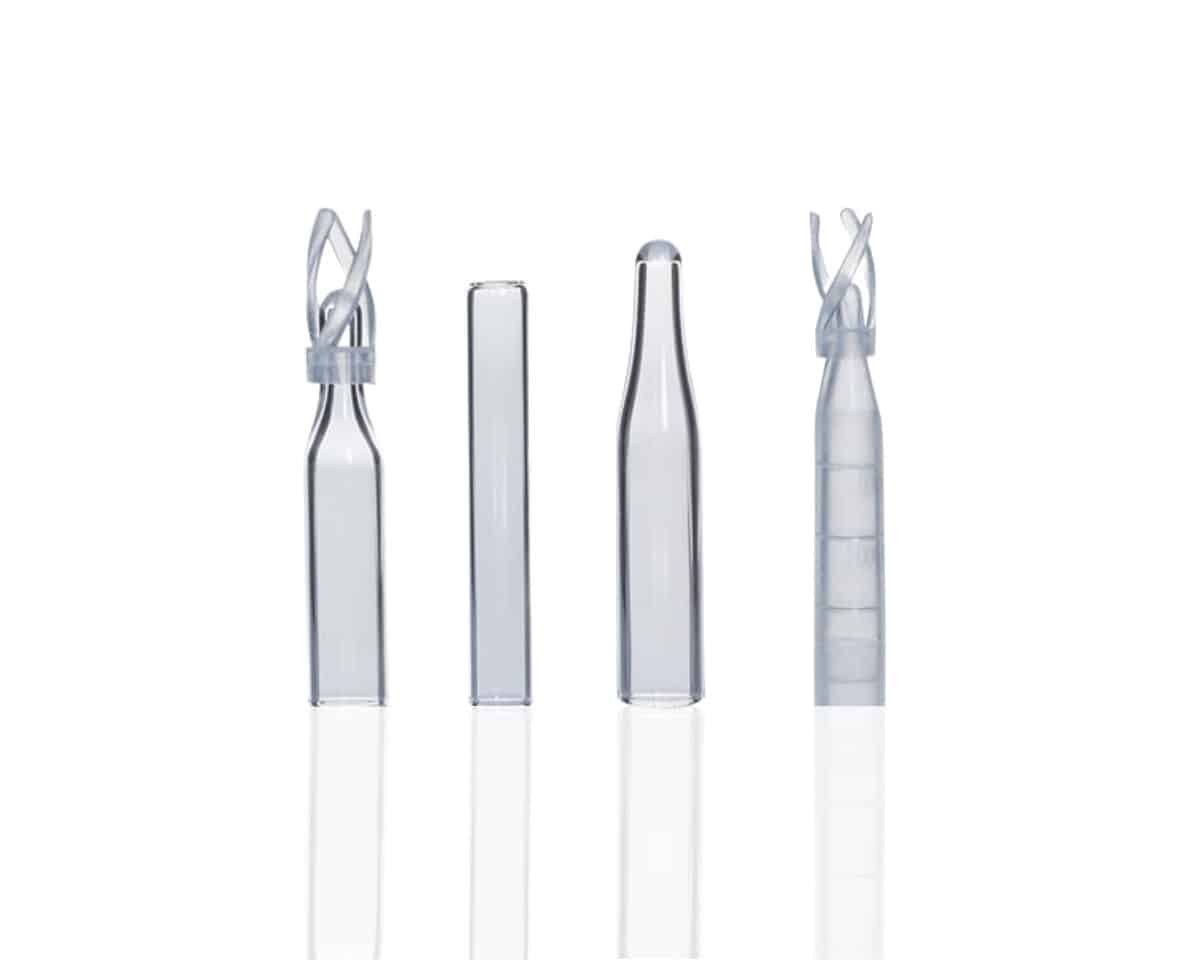
So, which one should you choose? Well, it depends on your industry and what your priorities are. When it comes to selecting between sterilized and dust-free HPLC vials, here are a few key considerations:
When to Choose Sterilized HPLC Vials
If your application involves sensitive biological samples or pharmaceuticals that require absolute sterility, then sterilized vials are the way to go. For instance, if you’re dealing with samples for microbiological analysis, clinical research, or vaccine storage, even a small chance of contamination could compromise your results or pose health risks. Sterilized HPLC vials undergo rigorous cleaning and sterilization processes, ensuring that all microbial life forms are eliminated.
Imagine running an HPLC analysis on a pharmaceutical sample that needs to be injected into a patient. Here, sterility is a must. Even trace microbial contamination could lead to false results or even cause harm to the patient. In such scenarios, sterilized vials give you peace of mind and the assurance that you’re working with uncontaminated containers.
When to Choose Dust-Free HPLC Vials
For most applications, especially in chromatography, dust-free HPLC vials are sufficient. If you’re analyzing chemicals, solvents, or conducting environmental testing where the risk of biological contamination is minimal, a dust-free vial provides the level of cleanliness you need. These vials are manufactured and packaged in clean environments to ensure there’s no dust, dirt, or particles that might interfere with your analysis.
Think about routine HPLC analyses in a research lab or chemical plant. The primary concern here isn’t microbial life but rather the presence of tiny particles that could impact your readings. Dust-free vials are designed to keep these particulates out without the extra cost and procedures associated with sterilization.
Is Dust-Free Enough in Most Cases?
In the world of HPLC, dust-free packaging is often more than enough to ensure accurate and reliable results. Since many chromatography applications don’t require absolute sterility, opting for dust-free vials helps you maintain a clean environment without unnecessary costs. These vials are specifically designed to eliminate any particulates that could interfere with sample integrity or equipment performance. So, unless you’re working in a sterile or clinical setting, dust-free is generally the go-to choice.
In summary, if you’re focused on chemical analyses, solvent testing, or environmental studies, dust-free vials usually suffice. But if your work involves biological or pharmaceutical applications where sterility is crucial, go for the sterilized option. It’s all about understanding the needs of your specific industry and the stakes involved.
When Dust-Free Packaging is Enough
If you’re working with electronics, automotive components, or non-sterile pharmaceuticals, dust-free packaging is probably sufficient. It’s cost-effective and efficient for keeping out those pesky particles that can ruin delicate machinery or cause cosmetic defects.
When Sterilization is Essential
In contrast, sterilization is a must when working with anything that could impact human health—medical devices, surgical tools, injectables, or even certain food products. Imagine the disaster if a syringe wasn’t sterile! It’s all about protecting the end user.
Real-World Examples and Case Studies
Let’s bring this to life with some real-world examples.
Electronics Industry
In the electronics industry, manufacturers use dust-free packaging to prevent particles from entering semiconductors and circuit boards. A tiny bit of dust could lead to malfunctions or short circuits, costing companies a lot of money.
Medical and Pharmaceutical Industry
In contrast, hospitals and pharmaceutical companies rely heavily on sterilization. Surgical instruments are sterilized using autoclaves, and medicines are packaged in sterile conditions to prevent contamination that could lead to infections.
Conclusion: Dust-Free or Sterile—Which Do You Need?
So, what’s the verdict? Dust-free packaging is great for maintaining a clean environment and preventing physical contaminants. But if you’re working in a field where microbial contamination could be life-threatening, then sterilization is the way to go.
Fianlly, if you’re on the lookout for high-quality HPLC vials at sensible pricing, you won’t miss out Mastelf. With over 13 years of experience in chromatography vials, we can help you find the exact vials you need for your applications.


Our expertise ensures that you get reliable and precise products tailored to your specific requirements. Whether you’re in pharmaceuticals, research, or any other industry relying on HPLC, we understand your needs and are here to support you in making the right purchase.
Reach out to Mastelf, and let us assist you in procuring the perfect vials for your work.