Choosing a suitable gas carrier can cause headaches. What things you should be careful about, what is essential, what is not…
Also, you might wonder what extra knowledge you should have for dealing with gas carriers for your gas chromatography analysis.
So, in this article, you will find a handful of information about choosing a suitable gas carrier. Read about specifications you should be careful about, and make the right decision for your gas chromatography.
What Are The Three Carrier Gasses You Can Use In Gas Chromatography?
Choosing a suitable gas carrier for your gas chromatography analysis can lead to many questions. But, the questions about which gas to use will be answered. Well, once you think about the aspects that will affect your research.
The parameters are:
- Price
- Performance
- Speed
- Analytical compatibility
- Availability
There are three gases used in gas chromatography (GC).
- Helium
- Hydrogen
- Nitrogen
Source: https://www.peakscientific.com
There are more, like Argon, which we will mention later in the text, but, the three most common ones are listed.
Helium is fast and safe for GC analysis, well, better than hydrogen in some aspects.
But… there is always a but!
Helium is a limited resource, and it is becoming more limited day by day. Now that the resources are limited, it is more expensive and not always available. That is why everybody is switching to the other two gases.
Hydrogen is an amazing and sufficient carrier gas in gas chromatography. It is an excellent choice for some situations because of its brief analysis. Also, it is a low-cost gas carrier and has optimal efficiency.
Scientists and researchers use hydrogen as a gas carrier for GC now more than ever because of the helium shortage. And the use of hydrogen is growing very fast because there are new instruments adapted for hydrogen every day.
It is flammable, but it can endure more rapid linear velocities, and researchers can have a quicker analysis.
Nitrogen is a delicate gas carrier. Scientists usually use nitrogen for packed columns and capillary GC.
Its linear velocities need to be slower than either helium or hydrogen to maintain efficiency. This leads to longer hours of analysis, and speed is critical.
It also has reduced sensitivity, but the most significant advantage of using nitrogen is its ability to produce the narrowest chromatographic peaks.
All in all, the three gases have a lot to offer.
But, how to choose the right one for your analysis? Let’s talk about that.
How To Choose Carrier Gas for Gas Chromatography?
Choosing which gas carrier you should utilize depends on so many things. For example, it depends on your applications and the detector you’re using.
Which One to Use for Packed Columns?
Remember when we said we would mention argon?
Well, for packed columns, you can use helium, nitrogen, and argon.
Helium’s advantage is that it can be removed with a separator in gas chromatography-mass spectrometry (GC-MS).
On the other hand, nitrogen’s advantage is the cost which is less than what you would pay for helium and argon.
And as mentioned, there is argon gas. It is beneficial if you want to analyze gas for helium and hydrogen content by TCD. Use it for isothermal GC. Also, use it with electron capture detection.
Which One to Use for Capillary Columns?
The best choice here would be helium. It is:
- inert,
- won’t set on fire like others, and
- has specific physical properties.
Those properties give helium high resolution.
The next obvious choice is hydrogen. Hydrogen is, as we know it, inflammable. But, its high diffusivity will provide the ability for rapid linear velocities.
Also, hydrogen analysis is faster but with the same separation efficiency as helium. Short research = increased throughput = lower costs per sample!
Capillary Columns – Van Deemter Plot
Learn more about the Van Deemter equation here.
The graph below demonstrates the connection between “HETP” and the carrier gas’s average linear velocity under isothermal circumstances.
HETP (height equivalent of a theoretical plate) is one measure of column efficiency.
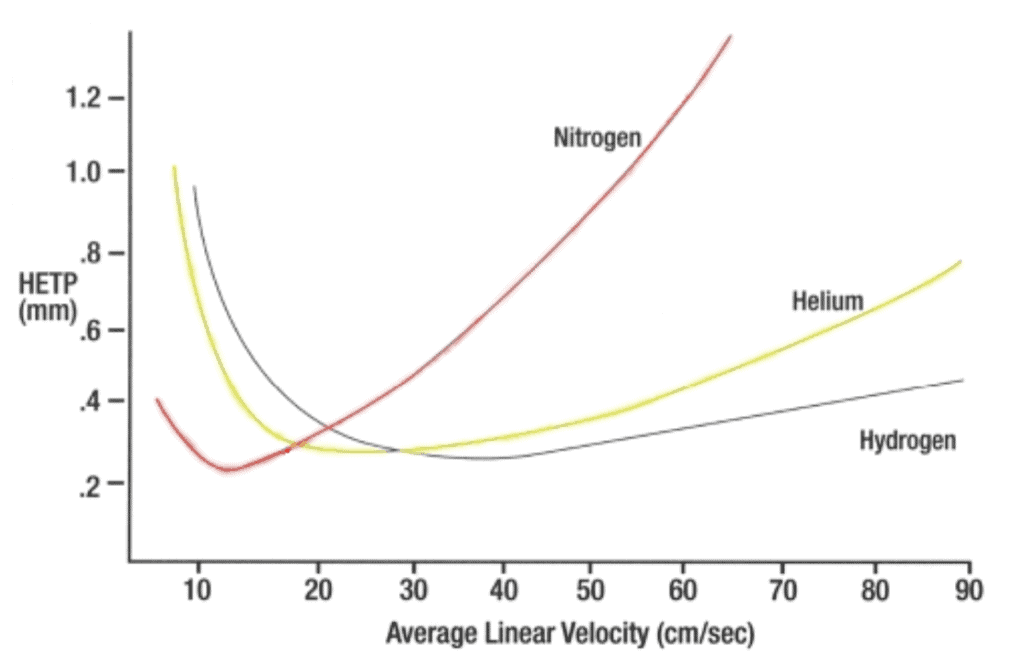
The type of the carrier gas and its linear velocity affect HETP (not volume flow rate). The separation is more efficient when the HETP is smaller.
When you adjust the carrier gas linear velocity to the value where HETP is the lowest, you may get the maximum possible efficiency (optimum separating power) from your GC.
The optimal rate for the system described above is 12 cm/sec (nitrogen). Also, it is 20 cm/sec for helium, and 36 cm/sec for hydrogen.
But, nitrogen is not the same as helium or hydrogen.
Now let’s check the nitrogen.
Although nitrogen has a lower minimum than the other two gases, its curve becomes steeper as linear velocity increases.
Nitrogen is the least efficient of the three gases as a carrier gas for temperature-programmed chromatography*. That is because modest variations in linear velocity during a run can result in considerable efficiency deterioration.
The efficiency loss that is shown in the picture caused carrier gas linear velocity. It dropped during temperature program when the carrier was not controlled.
In contrast, the minimal HETP with hydrogen is unaffected by considerable variations in linear velocity. Therefore, the linear velocity may be set anywhere between 30 and 60 cm/sec during the run without sacrificing separation efficiency.
Overall, hydrogen is the favoured capillary column carrier gas.
* The method of adjusting the column temperature during a run is temperature-programmed gas chromatography (TPGC). It is a popular approach for screening fresh samples and is particularly successful in optimizing an analysis. The majority of capillary GC techniques are temperature controlled.
There is more! Keep reading!
How To Switch From Helium To Hydrogen?
Hydrogen generated by a gas generator is a:
- safer,
- more consistent, and
- more dependable gas supply than cylinders.
Also, it is more convenient and environmentally friendly than helium. We all know what has been happening with helium for the past years.
This is a quick step-by-step guide for converting to hydrogen.
Step-by-Step Guide for Converting Carrier Gas
The first thing you should do is go over all the existing method data and instrument conditions.
You should make copies of all methods performed on the GC in the program. Then check the GC for leaks to ensure that the flow rates specified in the procedure are fulfilled when you complete your analysis.
Of course, verify that the GC fulfills all setpoints by measuring all flow rates specified in your technique (septum purge flow, split flow, carrier gas, and detection gases (if applicable)).
Then it would be best if you made a note of your carrier gas’s linear velocity. Take a sample and keep an eye on the system to confirm that the flow is consistent. You should write down if you see any changes in the flow.
And be sure to save an excellent chromatogram to compare findings after switching carrier gases.
The second thing you have to do before switching carrier gas is periodic GC maintenance. Replace your inlet septum. Use a low-bleed septum that your GC system recommends.
Then replace the lining. When using hydrogen carrier gas for GC analysis, a tapered liner can help. If you’re not already using tapered liners, it’s good to get some before you set up the system.
Also, depending on how old your gas purifiers are, replacing them with new ones may be necessary.
If you’re using a GC-MS and the ion source has to be cleaned, this is an excellent opportunity. Because hydrogen carrier gas should keep the ion source in better shape for longer than helium, you should notice that ion source cleaning is necessary less frequently once you’ve switched carrier gas.
If the column is still in good shape, you can use it.
The third step is to replace the tube.
Helium supply tubing should be removed. If you use the same tube to provide hydrogen, your analysis will get much background, and it will take longer to optimize.
If you’re converting from helium to nitrogen for makeup, you should also replace that tubing.
Use, for example, a 1/8″ laboratory-grade copper or stainless steel tubing within 3m (10 feet) of the application. If you’re piping gas from a distance larger than 3 meters, you may need to use a 14-inch tube and decrease it to 1/8-inch at each GC.
If you’re supplying a GC-MS, you’ll only need to run one line from the Hydrogen generator to the GC.
Don’t forget that you should be using compression fittings should for all connections.
Please do not use weld connections and sealing agents. These can pollute your system by introducing volatile organic compounds (VOCs) into the gas stream.
Step four is gas to the GC. According to the manufacturer’s site preparation recommendations, you should install the gas generators that feed your GC.
Using an electronic leak tester, make sure the connections to the GC are leak-free. Also, please do not use liquid leak detectors. They might contaminate your gas lines.
Conclusion
Choosing the right carrier gas can be tricky if you don’t have experience with it.
Hopefully, you’ve found the information you needed. If you still have questions, make sure to leave a comment! We will be more than happy to help!











